ORIGINAL
Reference values of parathyroid hormone and vitamin D Hormone by chemiluminescent automated assay
Valores de referencia de hormona paratiroidea y vitamina D por método quimioluminiscente automatizado en perros
Beatriz Martiarena,1* Esp, Nadia Kogovsek,5 Bioq, Helena Salerni,5 Esp, Víctor Castillo,2 Ph.D, Georgina Brandi,3 MV, Mariela Regonat,1 MV, Andrea Visintini,1 Esp, Héctor Quintana,4 MV, P Otero,5 Bioq.
Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Cátedra de Clínica Médica de Pequeños Animales y Hospital Escuela de Medicina Veterinaria, 1Servicios de Nefrología, 2Endocrinología y 3Laboratorio, 4Nutrición. Av. Chorroarrín 280, Ciudad Autónoma de Buenos Aires (CP1427) (C.A.B.A.), Argentina. 5Hospital General de Agudos “Carlos G.Durand” Servicio de Endocrinología, Av. Diaz Velez 5044 (CP 1405DCS) C.A.B.A.
*Correspondence: bmartiar@fvet.uba.ar
Received: July 2014; Accepted: January 2015.
ABSTRACT
Objective. Provide reference data for parathyroid hormone 1-84 (PTH 1-84) and 25OH Vitamin D (25OH D) using a new technique. Materials and methods. The hormones were evaluated, in serum, using a third generation automated chemiluminescent method for PTH in a group of 60 adult dogs, clinically healthy, grouped according to age in years in GA: 1 to 5, GB: 6 to 10 and GC:>10. Results. Data expressed as average ± DS were for PTH (pg/ml): 9.3±2.3; 12±6.3; 12.2±3.7; and for 25OH D (ng/ml): 84.2±27.8; 68.2±16.0; 63.6±23.1, respectively. The PTH value was significantly greater (p<0.05) in groups B and C in comparison with A, but no significant differences were observed between GB and GC. The 25OH D concentration was significantly less in GB (p<0.05) and in GC (p<0.01) in comparison with GA, showing no differences between GB and GC. A negative correlation between 25OH D and PTH was found (r= -0.28; p=0.015). Conclusions. Data contributed by this study provide reference values for PTH 1-84 and 25OH D, evaluated using a third generation automated chemiluminescent method for PTH in local dogs. The results will facilitate monitoring diseases that alter the metabolism of calcium and phosphorus in dogs
Key words: Calcium, phosphorus, parathyroid hormone, metabolism, vitamin D (Source: MeSH).RESUMEN
Objetivo. Aportar datos de referencia, para hormona paratiroidea 1-84 (PTH 1-84) y 25OH Vitamina D (25OH D) con una nueva técnica. Materiales y métodos. Las hormonas fueron valoradas, en suero, por un método quimioluminiscente automatizado, y de tercera generación para la PTH, en una población de 60 perros adultos clínicamente sanos, agrupados según edad en años en GA: 1 a 5, GB: 6 a 10 y GC:>10. Resultados. Los datos expresados como media ± DS fueron para la PTH (pg/ml): 9.3±2.3; 12±6.3; 12.2±3.7; y para la 25OH D (ng/ml): 84.2±27.8; 68.2±16.0; 63.6±23.1, respectivamente. El valor de PTH fue significativamente mayor (p<0.05) en los grupos B y C con respecto al A, pero no se observó diferencia significativa entre GB y GC. La concentración de 25OH D fue significativamente menor en el GB (p<0.05) y en el GC (p<0.01) con respecto al GA, sin haber diferencias entre los GB y GC. Se encontró una correlación negativa entre 25OH D y PTH (r= -0.28; p=0.015). Conclusiones. Los datos aportados en este trabajo permiten disponer de valores de referencia de PTH 1-84 y de 25OH D, valorados por un método quimioluminiscente automatizado y de tercera generación para la PTH, en una población local de perros. Los resultados facilitarán el seguimiento de enfermedades que alteran el metabolismo del calcio y fósforo en perros.
Palabras clave: Calcio, fósforo, hormona paratiroidea, metabolismo, vitamina D (Fuente: MeSH).
INTRODUCTION
The parathyroid hormone (PTH) is the one that controls the concentration of ionic calcium (iCa) in blood and extracellular liquid. Binding to membrane receptors on bone and kidney, it triggers a response that increases iCa; also, it stimulates the renal synthesis of 1.25 (OH)2D3 (Calcitriol), the active form of Vitamin D. This acts through the specific receptors for Vitamin D (VDR) in the intestines; increasing the absorption of dietary calcium in bones and kidneys to induce the flow of calcium towards the blood.
The resulting increased serum calcium and 1.25 (OH)2D3 exert a negative control on PTH synthesis and secretion in parathyroid cells. The increased blood phosphorus produces significant increases in the secretion of PTH (1,2). Dogs ineffectively photosynthesize Vitamin D through their skin, so that concentration depends on dietary intake and absorption through the intestines. Transported to the liver, it becomes 25-hydroxyvitamin D (25OH D or Calcidiol), to be later hydroxylated in the renal proximal tubule to calcitriol via stimulation by PTH (2.3).
PTH is a peptide molecule with 84 amino acids, synthesized in cells of the parathyroid gland as a precursor of 115 amino acids called pre-pro-PTH. The pre and pro sequences are unfolded and degraded during their transit through the cisterns of the endoplasmic reticulum, and the hormone is stored in two types of secretory vesicles: some contain the intact molecule with 84 amino acids (PTH 1-84) which it is the biologically active form, and the others contain proteases that degrade the molecule generating different carboxyl-terminal fragments (PTH Ct) (5,6). According to the extracellular concentration of iCa, parathyroid cells can secrete both PTH 1-84 and PTH Ct. iCa levels in the extracellular fluid are maintained within normal limits by a sensor calcium receptor (Ca-SR) coupled to a G protein located in the membrane of parathyroid cells. Low concentrations of extracellular iCa stimulate the secretion of PTH 1-84, and high levels of iCa activate Ca-SR which initiates an intracellular signaling cascade that culminates in the inhibition of biologically active PTH secretion and increases PTH proteolysis. Accordingly, by increasing the concentration of extracellular iCa, the proportion of PTH Ct fragments secreted are increased (2,3-5). While it was believed that these fragments had no biological activity, it has been demonstrated in humans and animals that some PTH Ct fragments have an opposite effect on blood concentration of iCa (5-7).
PTH in plasma circulates at very low concentrations and the presence of circulating peptide fragments makes it difficult to measure. This situation presents a wide variability between methods of measurement.
The first generation of PTH analysis was done with radioimmunoassay, and the few sensitive and specific studies measured both the intact form and their fragments. Second generation immunoassays, called intact PTH analysis, have a system of two antibodies, one specifically for the carboxyl terminal portion and one for the amino terminal portion of the molecule. Initially it was believed that these methods only detected PTH 1-84. However, later studies showed that there are circulating PTH fragments which, being degraded in the amino terminal portion of the molecule, do not possess amino acids 1-7 that are responsible for the biological activity of the hormone. This PTH 7-84 molecule and other long PTH Ct fragments can be detected by some second generation immunoassay with different specificity. This produces a great variation in the results obtained using different methodologies, especially in samples of patients with kidney disease, which have higher proportion of serum PTH Ct due to decreased renal clearance (1,2,5,7).
In recent years third generation immunoassays were developed that are more specific, and they do not detect PTH 7-84 fragments since the antibodies used in their design are directed against amino acids 1-7 of the molecule, so they confer less variability in measuring biologically active PTH (1,2,5-7).
There are no previous reports of reference values in dogs with automated chemiluminescent method for 25OH D and PTH, and the latter with new third generation technology. The aim of this study was to measure both hormones in a population of clinically healthy adult dogs to obtain reference values.
MATERIALS AND METHODS
Study population. Sixty clinically healthy dogs were studied, both sexes, older than one year, between 5 and 50 kg of weight. The animals came from the city and province of Buenos Aires (latitude: 34°30’S, longitude: 58°26’W, altitude: 25 m.a.s.l.), they lived in spacious kennels with access to a yard and garden. The geoclimatic condition at the time the study was during the month of August (austral winter). The average amount of daily sunlight was 10 h 25 min, with an average of 10 h 04 min, an average heliophany of 4.2 h/day and average temperature 12.0±0.8°C (source: National Meteorological Service of Argentina). They were fed commercial feed with an adequate intake of calcium and phosphorus for their age and body condition.
Experimental design
Selecting clinically health animals. The criteria used were the absence of clinical signs, normal physical examination, and the evaluation of urine, blood count and serum biochemistry within the laboratory references.Formation of study groups. They were divided into three groups according to age range in years: Group A (GA): 1-5, Group B (GB) of 6-10 and group C (GC):> 10. While each was formed of 10 males and 10 females, the data were analyzed without distinction by sex, because a preliminary assessment, at this writing, found no gender differences in age groups.
Collection and processing of samples for urine, general and endocrine biochemistry. Blood and urine samples were collected solids, 12 h fasting, in the morning (8 and 10 am). To determine the normality of the animals studied, a full analysis of total urine, protein / creatinine ratio of urine (UP/C), hemogram and serum biochemical measurements (with automated enzymatic colorimetric methods) of: total protein, albumin, urea, creatinine, AP, GPT (ALT) and GOT (AST), total calcium (Ca), inorganic phosphate (Pi) was done. The Ca total value was corrected with the albumin by formula (Ca - Albumin + 3.5).
Measuring PTH and Vitamin D. The blood of each dog was centrifuged within 30 minutes of collection, and the serum was stored at - 20°C until processed. The PTH 1-84 measurement was by third generation chemiluminescence (DiaSorin Liaison) with functional sensibility: 4.0 pg/ml; 100% specificity for PTH 1-84 and 0% crossed reactivity for PTH 7-84 and other fragments; with coefficient variation (CV) intra-essay at 6% and CV inter-essay at 9%.
The total 25OH D measurement was also done by chemiluminescence (DiaSorin Liaison) with a functional sensibility of 4.0 ng/ml, 100% specificity for 25-OH Vitamin D2 and 25-OH Vitamin D3 and 1.3% crossed reactivity with 3-epi 25-OH Vitamin D3; CV intra-essay at 3.9% and CV inter-essay at 9.8%.
Processing site. Samples for general biochemistry were processed at the Teaching Hospital Laboratory, Faculty of Veterinary Medicine, University of Buenos Aires. The hormone measurement was performed at the Laboratory of the Endocrinology Division of the Hospital General de Agudos Carlos G. Durand.
Ethic approval. The Committee on Animal Welfare of the Faculty of Veterinary Medicine, UBA, approved the CICUAL N 2013/3 procedure subsidized by UBACyt 2011/2014 V-05.
Statistical analysis. The distribution of variables was analyzed with the D’Agostino and Pearson Test. Because PTH and Pi did not show a normal distribution, all variables were normalized transforming them into the logarithmic form, allowing, in this way, using one way ANOVA parametric tests followed by the Bonferroni test for multiple comparison of means to compare the three age groups. The variables were correlated using Pearson’s test. To obtain minimum and maximum values, 3 and 97 percentiles were calculated.
The values are expressed in natural form (anti logarithmic) as mean ± DS, and the minimum values (percentile 3) and maximum (Percentile 97). The selected level of meaning was p<0.05.
RESULTS
The values obtained for calcium (mg/l) were: median 10.9, with a range between 8.8 and 12, and for phosphorus (mg/dl) median 4.2, with a range between 2.2 and 5.5. They remain within the reference values for the laboratory, and no differences between age groups (p = 0.1 Ca and Pi, p = 0.3) were found.
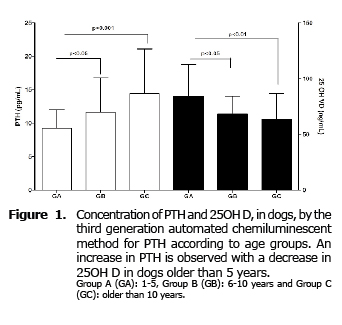
For PTH significant differences between the three groups studied (p=0.04) were found, increasing significantly (p≤0.05) in groups B and C compared to GA. No differences between GB and GC (Figure 1) were found.
For 25OH D significant differences were also found between the three groups (p = 0.005). Contrary to what happens with PTH, the concentration of this hormone decreases significantly in GB (p≤0.05) and GC (p≤0.01) compared to GA, with no difference between groups B and C (Figure 1).
The 25OH D had a negative and weak correlation with the PTH in a significant way (r= -0.28; p=0.015) (Figure 2).
No correlation was observed between the hormones studied and the concentration of Ca and Pi.
PTH and 25OH-D values, expressed as median and minumun-maximun according to the group, are showed in the table 1.
DISCUSSION
The few publications that evaluate PTH in clinically healthy dogs were performed with different methodologies than those used in this study. Some used IRMA (8.9) and other second-generation chemiluminescence (using a system of two antibodies, one directed against an epitope in the Ct region and another against the Nt region of the molecule (amino acids 1-34), measuring intact PTH and NO fragments 1-84) (1,8,7,10-12).
In this study an automated third generation chemiluminescence method was used (Ac directed against the Nt region of PTH, specific for amino acids 1-4 of the molecule, this allows avoiding cross-reactions with PTH peptides that have the same biological activity, particularly PTH 7-84, so only intact PTH values are evaluated) (13). The quality of avoiding interference of the fragments make it of special interest in chronic renal failure, as they accumulate in the blood by decreasing glomerular filtration, since the kidney is responsible for cleansing the urine (2,6,7).
A third generation model was used by Machado and Moutinho (14) in dogs, but they concluded that it was not useful to evaluate PTH. Pineda et al (15) were able to measure it in cats with a manual technique, which required 2 successive measurements per sample.
The discrepancy in the results reported by other authors and in this study result from inter-method variability that cannot be correctly compared. However, they provide higher maximum values than this study, possibly due to not only measuring active PTH (8-14).
The decrease in glomerular filtration that occurs with age decreases urine phosphorus elimination, and consequently it increases in the blood, followed by a decrease in serum calcium. Although both ions are kept within the range of reference serum, this situation stimulates the synthesis and secretion of PTH. This physiological variation explains the slight increase in PTH levels observed in dogs older than 5 years (GB and GC) and agrees with the results found by Aguilera-Tejero et al (8) in a study with another methodology PTH and found in a population of clinically healthy dogs over 10 years.
PTH results obtained in this study are similar to those reported in human medicine with the same method, which also observed higher values in the elderly such as those found in GB and GC (2, 16).
The variability in PTH results is related to the clinical condition of the patient (primary and secondary conditions of the parathyroid) and are based on pre-analytical and analytical biological conditioning. The first is inherent in handling samples, considering that circulating PTH concentration varies throughout the day (circadian rhythm, intake of calcium and phosphorus, etc.) (1,2,5,8,17). To minimize these changes, it is advisable to remove the blood sample at the same time of day and under the same conditions. Also, there are differences in the results of PTH according to the serum, plasma, type of anticoagulant, time and storage method of the sample. Intact PTH concentration decreases starting at 24 h at temperatures of 18-25°C (1,2).
Veterinary measurements with a second generation method were performed on plasma (8-10,18). In this study, pre-analytical conditions that guarantee the stability of the sample from blood collection to processing were established, thereby minimizing errors inherent in sample preservation.
The level of vitamin D sufficiency is evaluated in human medicine, through 25OH D, with an expected desirable value, at present, greater than 30 ng/ml (reserving 1-25 OH D measurements to exceptional cases), and is considered insufficient between 20 and 29 (commonly found in older adults ≥ 70 years) and poor <19 ng / ml (2.19). A similar situation was observed in our study where values > 30 ng/ml were found in groups A and B, with the lowest value (3rd percentile) below 30 ng/ml for the group C. This finding determined that the lower value in dogs older than 10 years is 20 ng / ml; or three dogs that were within this value could be considered insufficient. The few studies that evaluate vitamin D in dogs found no differences with age (8), although the method of measurement was different to that used in the present work.
The lowest concentration of 25OH D observed in elderly humans is explained by skin aging and less exposure to the sun, among other causes (2). This explanation would not be extrapolated to dogs because they ineffectively photosynthesize vitamin D in their skin, so that its concentration depends on dietary intake and absorption through the gut (18-20). In any case, our study population had adequate sun exposure. All the dogs ate the same food to avoid variations in intake, which is why intestinal absorption dysfunction would be a possible cause of the differences found between groups. The reduced concentration of 25OH D in older dogs in the study population was reflected by increased PTH to maintain calcium and phosphorus metabolism, since the decline in vitamin D decreases the inhibitory effect on PTH (1,2).
In conclusion, the contribution of PTH reference intervals with a third generation method and 25OH D in dogs, both by automated chemiluminescent technique, will be used to study diseases that alter calcium and phosphorus metabolism. This is the first report classified by age and where both hormones are correlated. In dogs older than 5 years, it is expected to find values of calcium and phosphorus in normal reference ranges with a physiological increase of PTH to counteract declining 25OH D.
Acknowledgment
Project CV-05, subsidized by UBACyT 2011/2014.
REFERENCES
1. Feldman E, Nelson R. Hipercalcemia e hiperparatiroidismo primario, hipocalcemia e Hipoparatiroidismo. Endocrionología y Reproducción Canina y Felina, 3ed, Editorial Intermédica 2007.
2. Cannata AJ. Alteraciones del metabolismo óseo y mineral en la enfermedad renal crónica: avances en patogenia, diagnóstico y tratamiento. España: Wolters Kluwer Health España, S.A; Lippincott Williams & Wilkins; 2010.
3. Schropp K. Phosphorous and phosphate metabolism in veterinary patients. J Vet Emerg Crit Care 2006; 17:127-134.
4. How KL, Hazewinkel H, MOL JA. Dietary Vitamin D Dependence of Cat and Dog Due to Inadequate Cutaneous Synthesis of Vitamin D. Gen Comp Endocrinol 1994; 96(1):12-18.
5. Friedman PA, Goodman WA, PTH (1–84)/PTH (7–84): a balance of power. Am J Renal Physiol 2006; 290:F975–F984.
6. Murray TM, Rao L, Divieti P, Bringhurst RF. Parathyroid Hormone Secretion and Action: Evidence for Discrete Receptors for the Carboxyl-Terminal Region and Related Biological Actions of Carboxyl- Terminal Ligands. Endocr Rev 2005; 26(1):78–113.
7. Sturgeon C, Sprague SM, Metcalfe W. Variation in parathyroid hormone immunoassay results–a critical governance issue in the management of chronic kidney disease. Nephrol Dial Transplant 2011; 26:3440–3445.
8. Aguilera-Tejero E, Lopez-Estepa JC, Mayyer-Valor R, Almadén Y, Concepción MT, Felsenfela AJ, Rodriguez M. Mineral metabolism in healthy geriatric dogs. Res Vet Sci 1998; 64:191-94.
9. Torrance AG, Nachreiner R. Intact parathyroid hormone assay and total calcium concentration in the diagnosis of disorders of calcium metabolism in dogs. J Vet Intern Med 1989; 3:86-89.
10. Torrance AG; Nachreiner R. Human parathormone assay for use in dogs: Validation, sample handling studies, and parathyroid function testing. Am J Vet Res 1989; 50:1123/1127.
11. Graham KJ, Wilkinson M, Culvenor J, Dhand NK, Churchera RK. Intraoperative parathyroid hormone concentration to confirm removal of hypersecretory parathyroid tissue and time to postoperative normocalcaemia in nine dogs with primary hyperparathyroidism. Aust Vet J 2012; 90(6):203-209.
12. Kathleen H, Greenfield C, Barger A, Schaeffer D, Ehrhart E, Pinkerton M, Valli V. Validation of a rapid parathyroid hormone assay and intraoperative measurement of parathyroid hormone in dogs with benign naturally occurring primary hyperparathyroidism. Vet Surg 2009; 38:122–132.
13. de La Piedra C, Fernández E, González Casaus L, González Parra E. Diferencias en la función de los péptidos paratiroideos. ¿Qué estamos midiendo? Nefrología 2008; 28(2):123-128.
14. Machado LHA, Moutinho FQ. Validação do método de quimioluminescência para determinação de PTH intacto em cães. Vet Zootec 2013; 20(1):84-90.
15. Pineda C, Aguilera-Tejeroa E, Raya A, Diez E, Rodriguez M, Lopez I. Feline parathyroid hormone: validation of hormonal assays and dynamics of secretion. Domest Anim Endocrinol 2012; 42:256–264.
16. Kogovsek N; Lacaze N; Theaux C; Salerni E; Mormando E; Astarita G; Bernatene D; Anzil O; Oero P. Comparación de tres metodologías para la medición de la Hormona Paratiroidea. Acta Bioquím Clín Latinoam 2012; 46(3):507-11.
17. López I, Aguilera-Tejero E, Estepa JC, Bas S, Mayer-Valor R, Jiménez A, Rodríguez M. Diurnal variations in the plasma concentration of parathyroid hormone in dogs. Vet Rec 2005; 157(12):344-347.
18. Suarez-Rey ML. Manejo de la enfermedad renal crónica. REDVET 2007; 2(1):1-18.
19. Mastaglia SR, Watson DZ, Oliver B. Controversia sobre los niveles adecuados de Vitamina D para la salud ósea propuestos por el instituto de medicina de los Estados Unidos y la comunidad médica internacional. Actual Osteol 2013; 9(2):207-16.
20. Allen T, Polzin D, Adams L. Renal Disease. Small Animal Clinical Nutrition, 4th edition. Chapter 19. Estados Unidos: Mark Morris Institute; 2000.
21. Bonagura JD, Twedt DC. Terapéutica Veterinaria Actual XIV. Madrid, España: Elsevier; 2009.